Publications
53.
Fujiyoshi, K.; Arii, S.; Yamatsugu, K.; Mitsunuma, H.; Kanai, M.*
“Photocatalytic phosphorylation of alcohols via oxidative activation of 1,4-dihydropyridine-N-phosphoramide”
Tetrahedron 2025, 189, 135008.
52.
Inoue, T.; Tomiya, D.; Fuki, M.; Kobori, Y.; Higashi, M.; Uesaka, K.; Yamanaka, A.; Kawashima, S. A.; Yamatsugu, K.; Mitsunuma, H.*; Kanai, M.*
“Azaanthraquinone PCET Catalysis Enables Chemoselective Decarboxylative Functionalization of Diverse Carboxylic Acids”
J. Am. Chem. Soc. 2025, 147, XXXX.
DOI: 10.1021/jacs.5c10807
51.
Ishihara, N.; Fukuoka, R.; Harada, S.; Yamatsugu, K.; Arai, S.
"Regio- and Stereoselective [3+2] cycloaddition of Isatogens with functionalized alkenes and its application to the synthesis of Halichrome A”
Tetrahedron Lett. 2025, 167, 155626.
50.
Liu, Z.; Xi, S.; McGregor, L. A.; Yamatsugu, K.; Kawashima, S. A.*; Sczepanski, J. T.*; Kanai, M.*
"A Method for Constructing Nucleosome Arrays with Spatially Defined Histone PTMs and DNA Damage”
Angew. Chem. Int. Ed. 2025, 64, e202500162.
49.
Nozaki, T.; Onoda, M.; Habazaki, M.; Takeuchi, Y.; Ishida, H.; Sato, Y.; Kujirai, T.; Hanada, K.; Yamatsugu, K.; Kurumizaka, H.; Kimura, H.; Kono, H.; Kawashima, S. A.*; Kanai, M.*
"Designer Catalyst-Enabled Regiodivergent Histone Acetylation”
J. Am. Chem. Soc. 2025, 147, 13732-13743.
DOI: 10.1021/jacs.5c01699
48.
Yamanashi, Y.; Takamaru, S.; Okabe, A.; Kaito, S.; Azumaya, Y.; Kamimura, Y. R.; Yamatsugu, K.; Kujirai, T.; Kurumizaka, H.; Iwama, A.; Kaneda, A.; Kawashima, S. A.*; Kanai, M.*
“Chemical catalyst manipulating cancer epigenome and transcription”
Nat. Commun. 2025, 16, 887.
47.
Fukuta, T.; Tatsumi, T.; Fujiyoshi, K.; Koyama, T.; Kawashima, S. A.; Mitsunuma, H.; Yamatsugu, K.*, Kanai, M.*
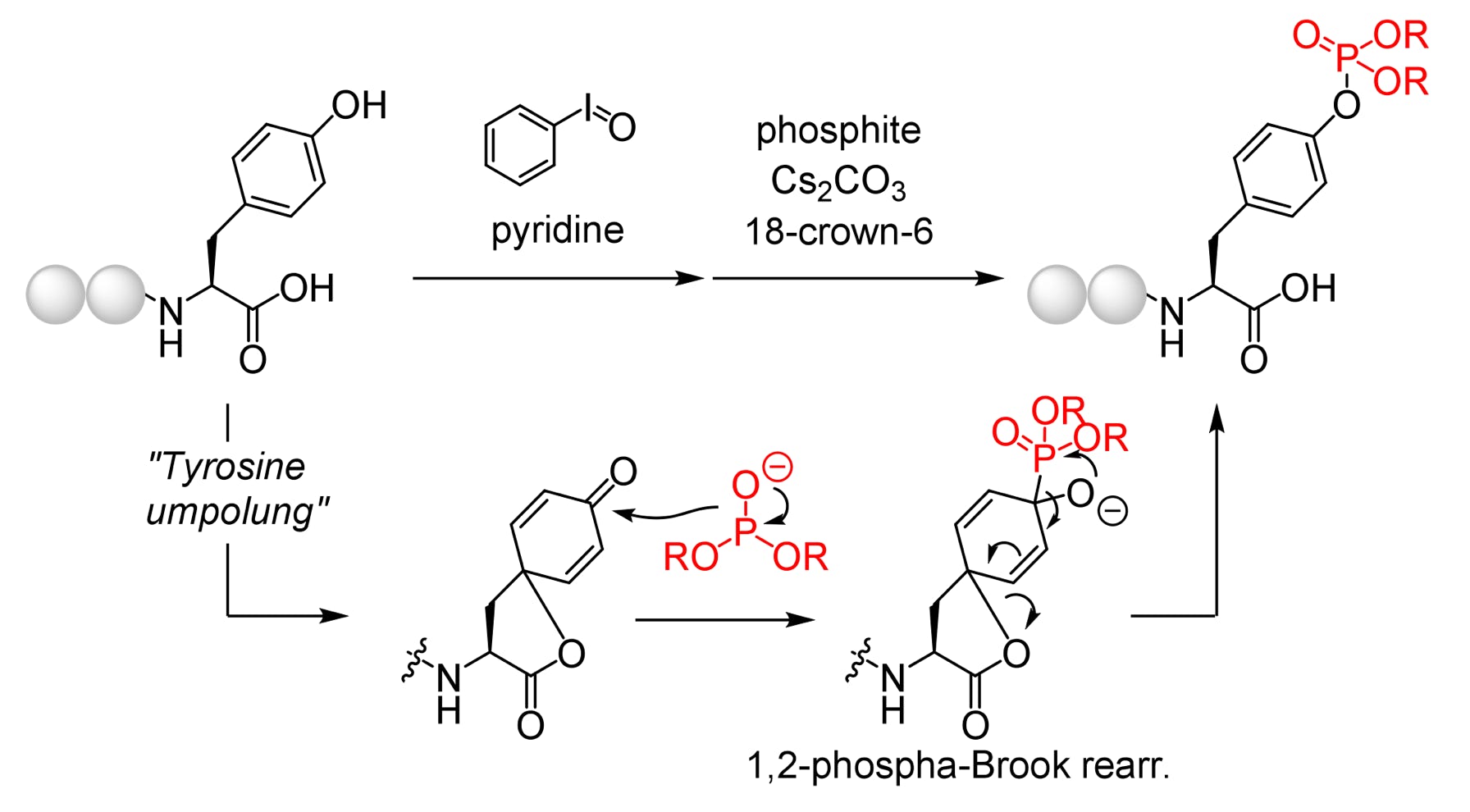
“Umpolung Phosphorylation of Tyrosine via 1,2-Phospha-Brook Rearrangement”
Org. Lett. 2024, 26, 41, 8827-8831.
46.
Tatsumi, T.; Zhao, S.; Kasahara, A.; Aoki, M.; Nishijima, K.; Ukon, N.; Kodama, T.; Takahashi, K.; Sugiyama, A.*; Washiyama, K.*; Yamatsugu, K.*; Kanai, M.*
“In Vivo-Stable Bis-Iminobiotin for Targeted Radionuclide Delivery with the Mutant Streptavidin”
Bioorg. Med. Chem. Lett. 2024, 108, 129803.

45.
Shimazoe, K.*; Donghwan, K.; Mineo, T.; Sato, T.; Ohta, S.; Tatsumi, T.; Sugiyama, A.; Yamatsugu, K.; Nomura, S.; Terabayashi, R.; Tomita, H.; Sonoda, T.; Shigekawa, Y.; Yokokita, T.; Uenomachi, M.
“pH dependence of perturbed angular correlation in DOTA chelated 111In measured with ring-shape gamma-ray detectors”
Interactions 2024, 245, 22.
44.
Fujimura, A.; Ishida, H.; Nozaki, T.; Terada, S.; Azumaya, Y.; Ishiguro, T.; Kamimura, Y. R.; Kujirai, T.; Kurumizaka, H.; Kono, H.; Yamatsugu, K.; Kawashima, S. A.*; Kanai, M.*
“Designer Adaptor Proteins for Functional Conversion of Peptides to Small-Molecule Ligands toward In-Cell Catalytic Protein Modification”
ACS Cent. Sci. 2023, 9, 11, 2115-2128.
43.
Sakata, J.; Tatsumi, T.; Sugiyama, A.*; Shimizu, A.; Inagaki, Y.; Katoh, H.; Yamashita, T.; Takahashi, K.; Aki, S.; Kaneko, Y.; Kawamura, T.; Miura, M.; Ishii, M.; Osawa, T.; Tanaka, T.; Ishikawa, S.; Tsukagoshi, M.; Chansler, M.; Kodama, T.; Kanai, M.; Tokuyama, H.*; Yamatsugu, K.*
“Antibody-mimetic drug conjugate with efficient internalization activity using anti-HER2 VHH and duocarmycin”
Protein Expr. Purif. 2023, 106375.
42.
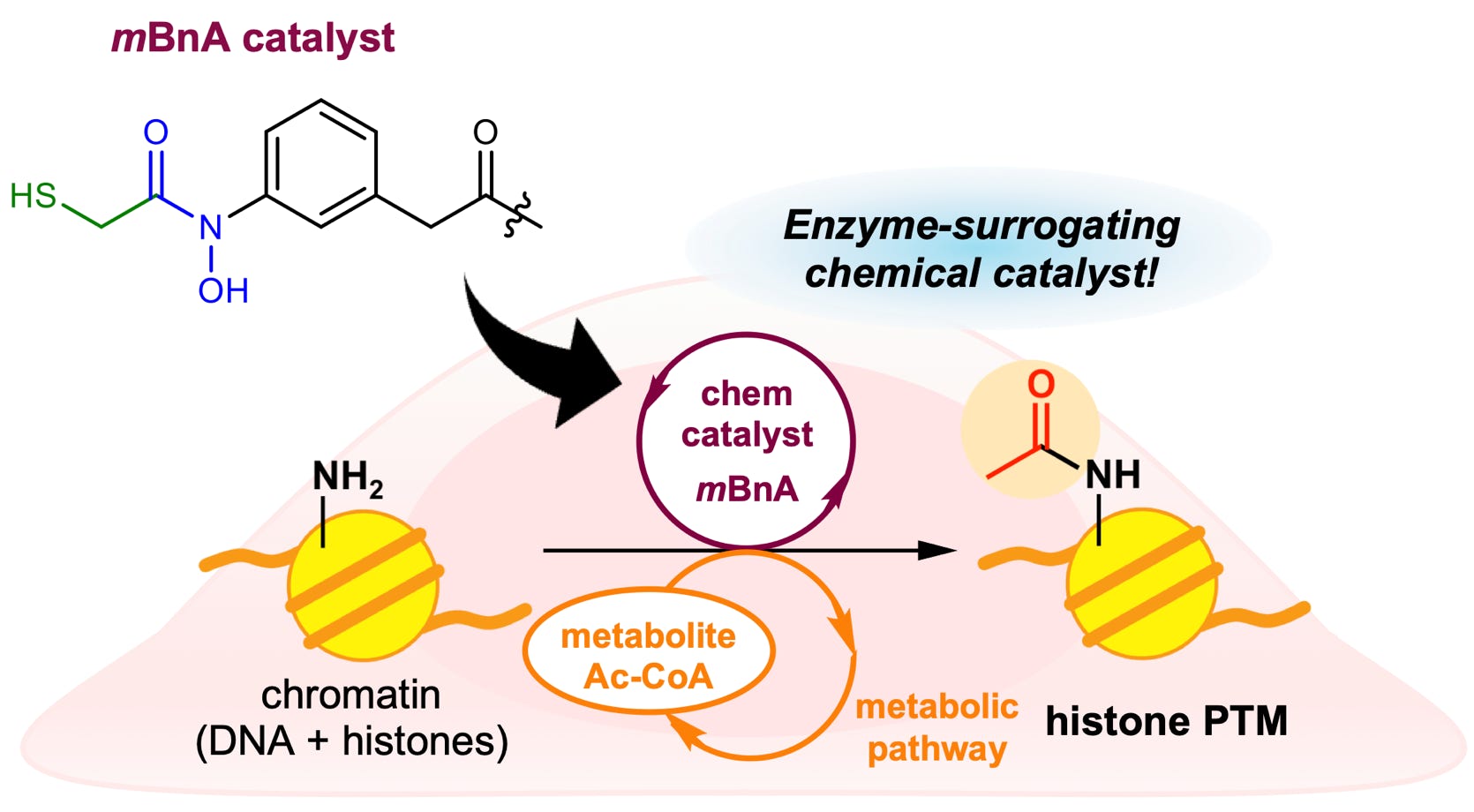
Habazaki, M.; Mizumoto, S.; Kajino, H.; Kujirai, T.; Kurumizaka, H.; Kawashima, S. A.*; Yamatsugu, K.*; Kanai, M.*
“A chemical catalyst enabling histone acylation with endogenous acyl-CoA”
Nat. Commun. 2023, 14, 5790.
41.
Mauro, E.; Lapaillerie, D.; Tumiotto, C.; Charlier, C.; Martins, F.; Sousa, S.; Métifiot, M.; Weigel, P.; Yamatsugu, K.; Kanai, M.; Munier-Lehmann, H.; Richetta, C.; Maisch, M.; Dutrieux, J.; Batisse, J.; Ruff, M.; Delelis, O.; Lesbats, P.; Parissi, V.*
“Modulation of the functional interfaces between retroviral intasomes and the human nucleosome”
mBio 2023, 14, 4, e01083-23.
40.
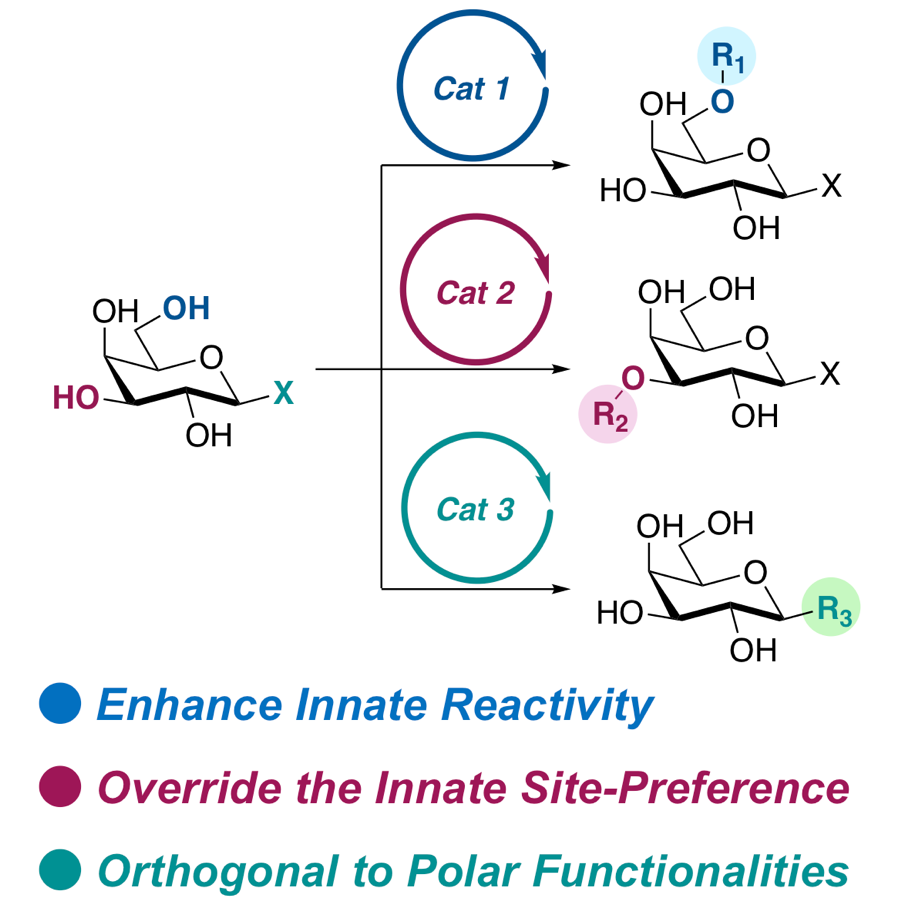
Yamatsugu, K.*; Kanai, M.*
“Catalytic Approaches to Chemo- and Site-Selective Transformation of Carbohydrates”
Chem. Rev. 2023, 123, 10, 6793–6838.
39.
Kaneko, Y.; Yamatsugu, K.; Yamashita, T.; Takahashi, K.; Tanaka, T.; Aki, S.; Tatsumi, T.; Kawamura, T.; Miura, M.; Ishii, M.; Ohkubo, K.; Osawa, T.; Kodama, T.; Ishikawa, S.; Tsukagoshi, M.; Chansler, M.; Sugiyama, A.*; Kanai, M.*; Katoh, H.*
“Pathological complete remission of relapsed tumor by photo-activating antibody–mimetic drug conjugate treatment”
Cancer Sci. 2022, 113, 4350-4362.
DOI: 10.1111/cas.15565
38.
Yamatsugu, K.; Katoh, H.; Yamashita, T.; Takahashi, K.; Sho, A.; Tatsumi, T.; Kaneko, Y.; Kawamura, T.; Miura, M.; Ishii, M.; Ohkubo, K.; Osawa, T.; Kodama, T.; Ishikawa, S.; Kanai, M.; Sugiyama, A.*
“Antibody mimetic drug conjugate manufactured by high-yield Escherichia coli expression and non-covalent binding system”
Protein Expr. Purif. 2022, 192, 106043.
37.
Adamson, C.; Kajino, H.; Kawashima, S. A.*; Yamatsugu, K.*; Kanai, M.*
“Live-Cell Protein Modification by Boronate-Assisted Hydroxamic Acid Catalysis”
J. Am. Chem. Soc. 2021, 143, 14976-14980.
DOI: 10.1021/jacs.1c07060

36.
Fujiyoshi, K.; Kawashima, S. A.; Yamatsugu, K.*; Kanai, M.*
“A Single-Step Asymmetric Phosphodiester Synthesis from Alcohols with Phosphoenolpyruvate Phosphodiester”
Synlett 2021, 32, 1135-1140.
DOI: 10.1055/a-1509-9275

35.
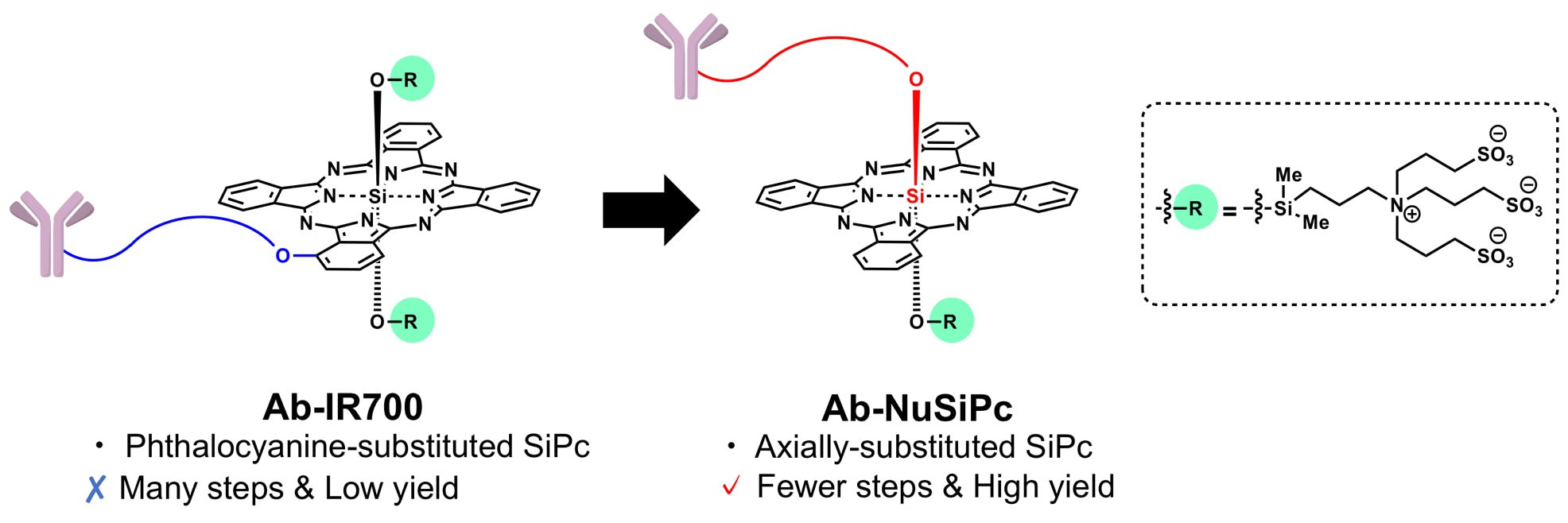
Takahashi, K.; Sugiyama, A.; Ohkubo, K.; Tatsumi, T.; Kodama, T.; Yamatsugu, K.*; Kanai, M.*
“Axially-substituted silicon phthalocyanine payloads for antibody-drug conjugates”
Synlett 2021, 32, 1098-1103.
DOI: 10.1055/a-1503-6425
34.
Fujiwara, Y.; Yamanashi, Y.; Fujimura, A.; Sato, Y.; Kujirai, T.; Kurumizaka, H.; Kimura, H.; Yamatsugu, K.*; Kawashima, S. A.*; Kanai, M.*
“Live-Cell Epigenome Manipulation by Synthetic Histone Acetylation Catalyst System”
Proc. Natl. Acad. Sci. USA 2021, 118, 4, e2019554118.

33.
Fujiyoshi, K.; Yamatsugu, K.; Kanai, M.
“POSOP”
Encyclopedia of Reagents for Organic Synthesis rn02368.
32.
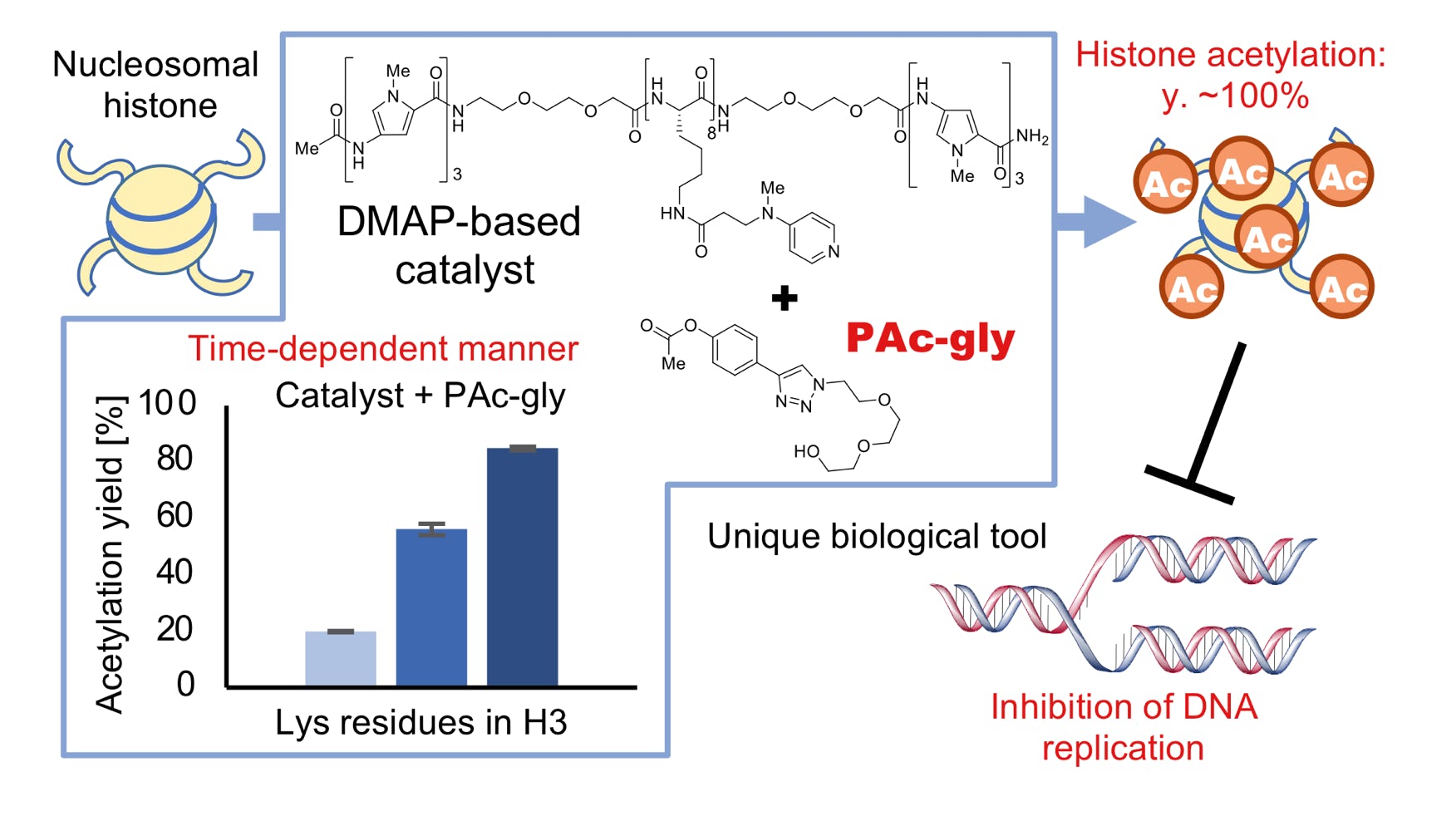
Kajino, H.; Nagatani, T.; Oi, M.; Kujirai, T.; Kurumizaka, H.; Nishiyama, A.; Nakanishi, M.; Yamatsugu, K.*; Kawashima, S. A.*; Kanai, M.*
“Synthetic hyperacetylation of nucleosomal histones”
RSC Chem. Biol. 2020, 1, 2, 56-59.
DOI: 10.1039/D0CB00029A
31.
Ohkawachi, K.; Kobayashi, D.; Morimoto, K.; Shigenaga, A.; Denda, M.; Yamatsugu, K.; Kanai, M.; Otaka, A.*
“Sulfanylmethyldimethylaminopyridine as a Useful Thiol Additive for Ligation Chemistry in Peptide/Protein Synthesis”
Org. Lett. 2020, 22, 14, 5289-5293.

30.
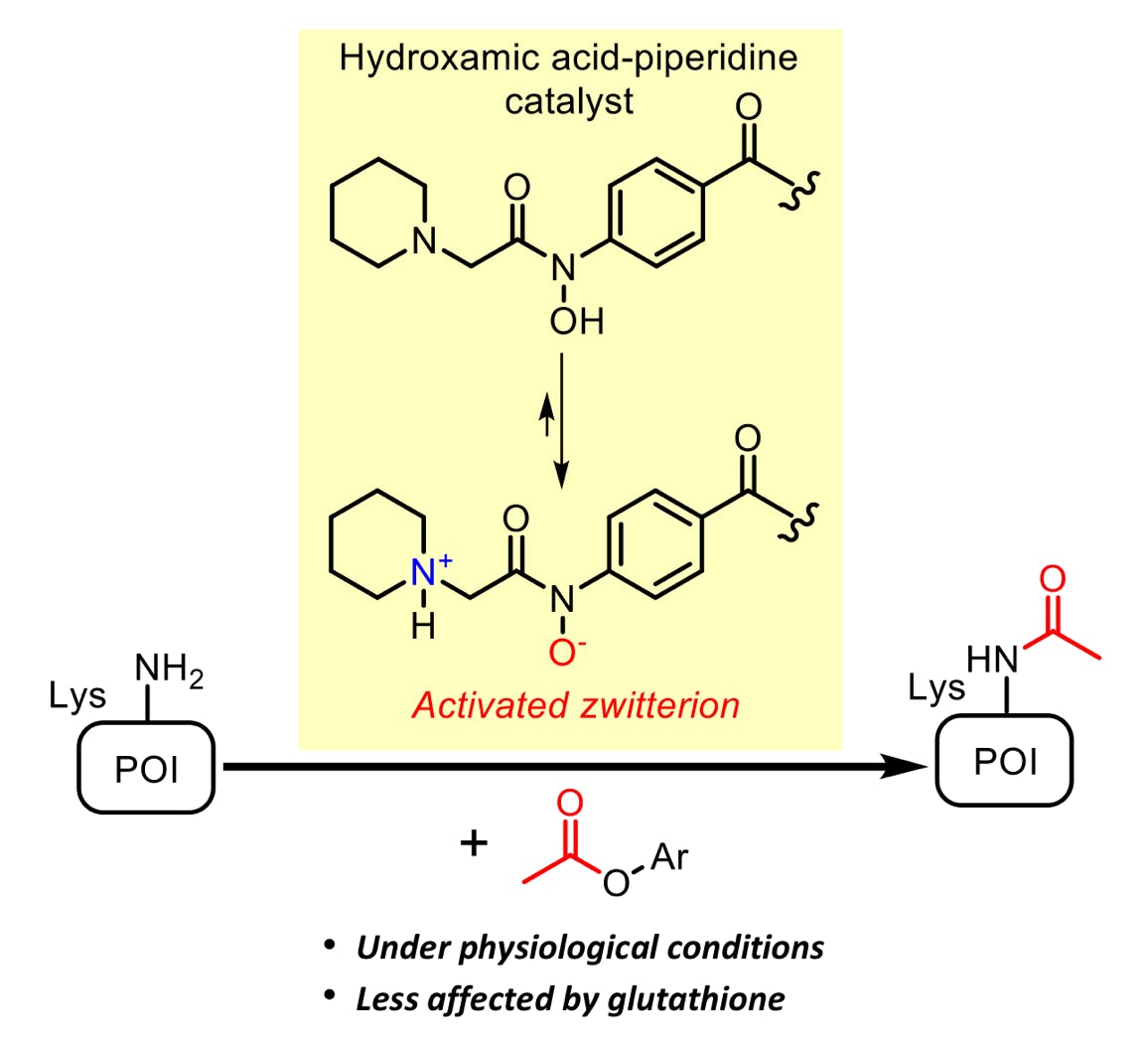
Mizumoto, S.; Xi, S.; Fujiwara, Y.; Kawashima, S. A.; Yamatsugu, K.*; Kanai, M.*
“Hydroxamic Acid-Piperidine Conjugate is an Activated Catalyst for Lysine Acetylation under Physiological Conditions”
Chem. Asian J. 2020, 15. 6, 833-839.
29.
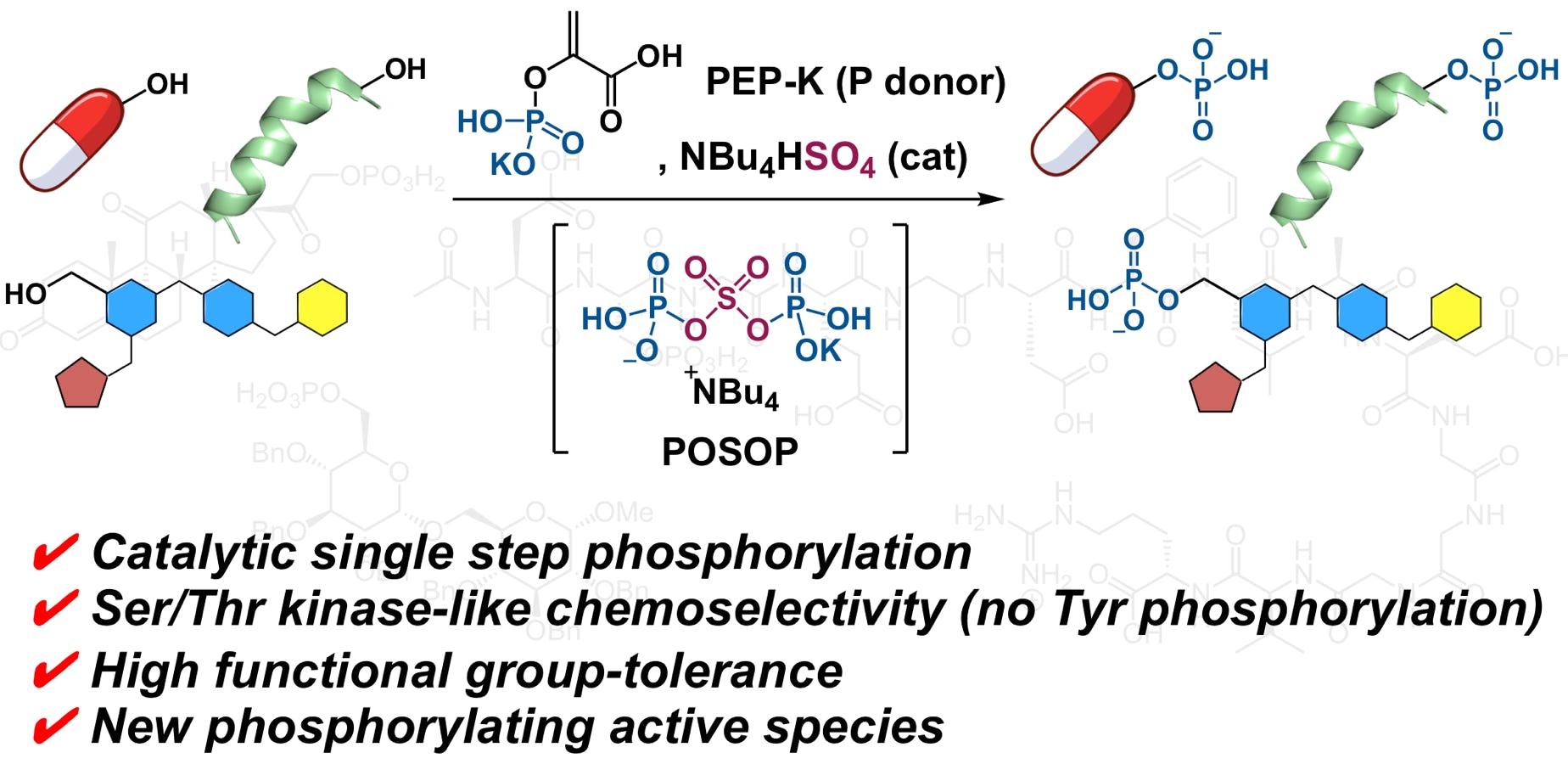
Domon, K.; Puripat, M.; Fujiyoshi, K.; Hatanaka, M.; Kawashima, S. A.; Yamatsugu, K.*; Kanai, M.*
“Catalytic Chemoselective O-Phosphorylation of Alcohols”
ACS Cent. Sci. 2020, 6, 2, 283-292.
28.
Ito, K.; Tatsumi, T.; Takahashi, K.; Shimizu, Y.; Yamatsugu, K.; Kanai, M.*
“A Stable and Cleavable O-Linked Spacer for Drug Delivery Systems”
Chem. Pharm. Bull. 2020, 68, 3, 212-215.
27.
Sugiyama, A; Kawamura, T.; Tanaka, T.; Doi, H.; Yamashita, T.; Shinoda, K.; Fujitani, H.; Yamatsugu, K.; Shimizu, Y.; Tatsumi, T.; Takahashi, K.; Kanai, M.; Mizohata, E.; Kawato, T.; Doi, T.; Inoue, T.; Kodama, T.*
“Cupid and Psyche system for the diagnosis and treatment of advanced cancer”
Proc. Jpn. Acad. Ser. B 2019, 95, 10, 602-611.
DOI: 10.2183/pjab.95.041
26.
Hamajima, W.; Fujimura, A.; Fujiwara, Y.; Yamatsugu, K.*; Kawashima, S. A.*; Kanai, M.*
“Site-selective synthetic acylation of a target protein in living cells promoted by a chemical catalyst/donor system”
ACS Chem. Biol. 2019, 14, 6, 1102-1109.

25.
Yamatsugu, K.*
“How My Experiences in Asymmetric Catalysis and Glycobiology Led to My Current Research in Synthetic Post-translational Modifications by Chemical Catalysts”
Yakugaku Zasshi 2019, 139, 2, 187-198.
24.
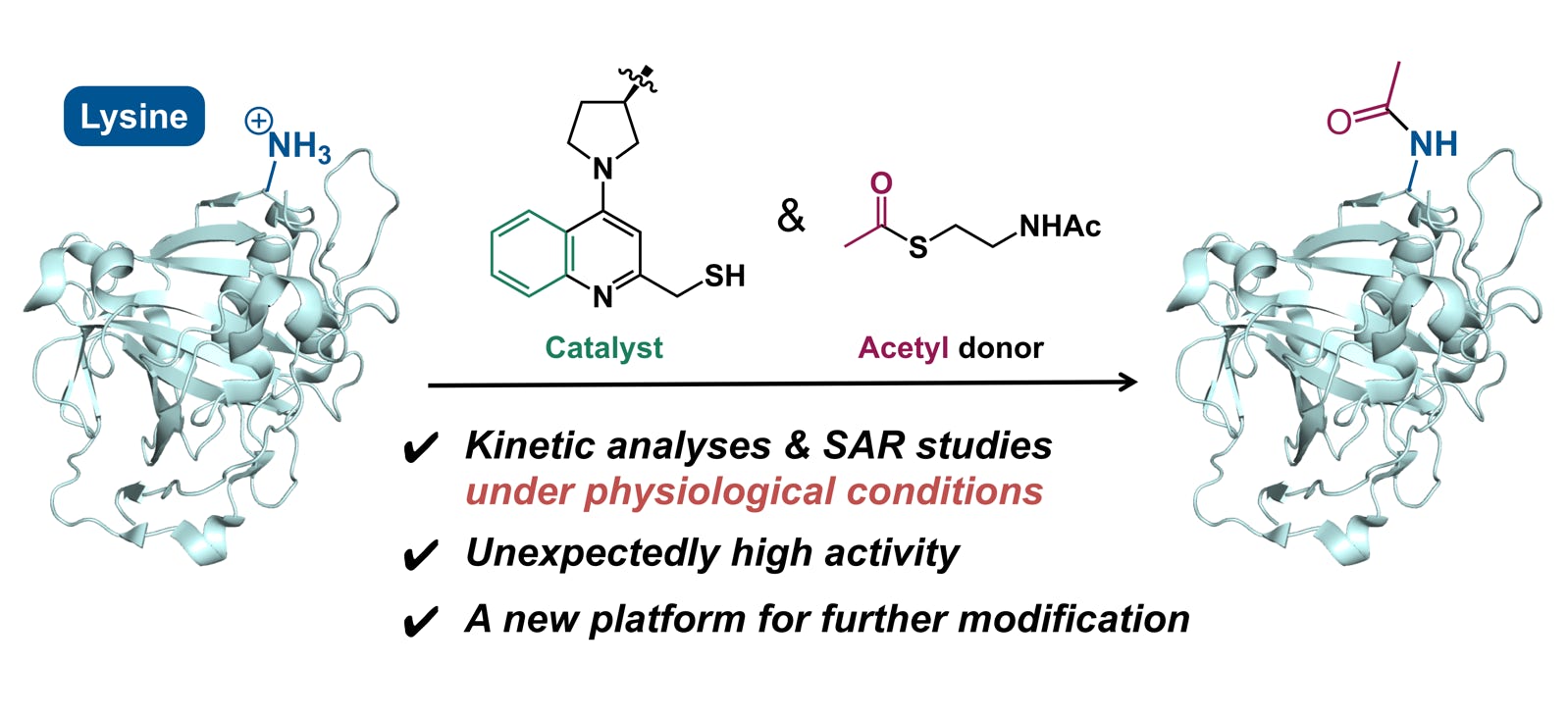
Yamatsugu, K.*; Furuta, M.; Xi, S.; Amamoto, Y.; Liu, J.; Kawashima, S. A.; Kanai, M.*
“Kinetic analyses and structure-activity relationship studies of synthetic lysine acetylation catalysts”
Bioorg. Med. Chem. 2018, 26, 19, 5359-5367.
23.
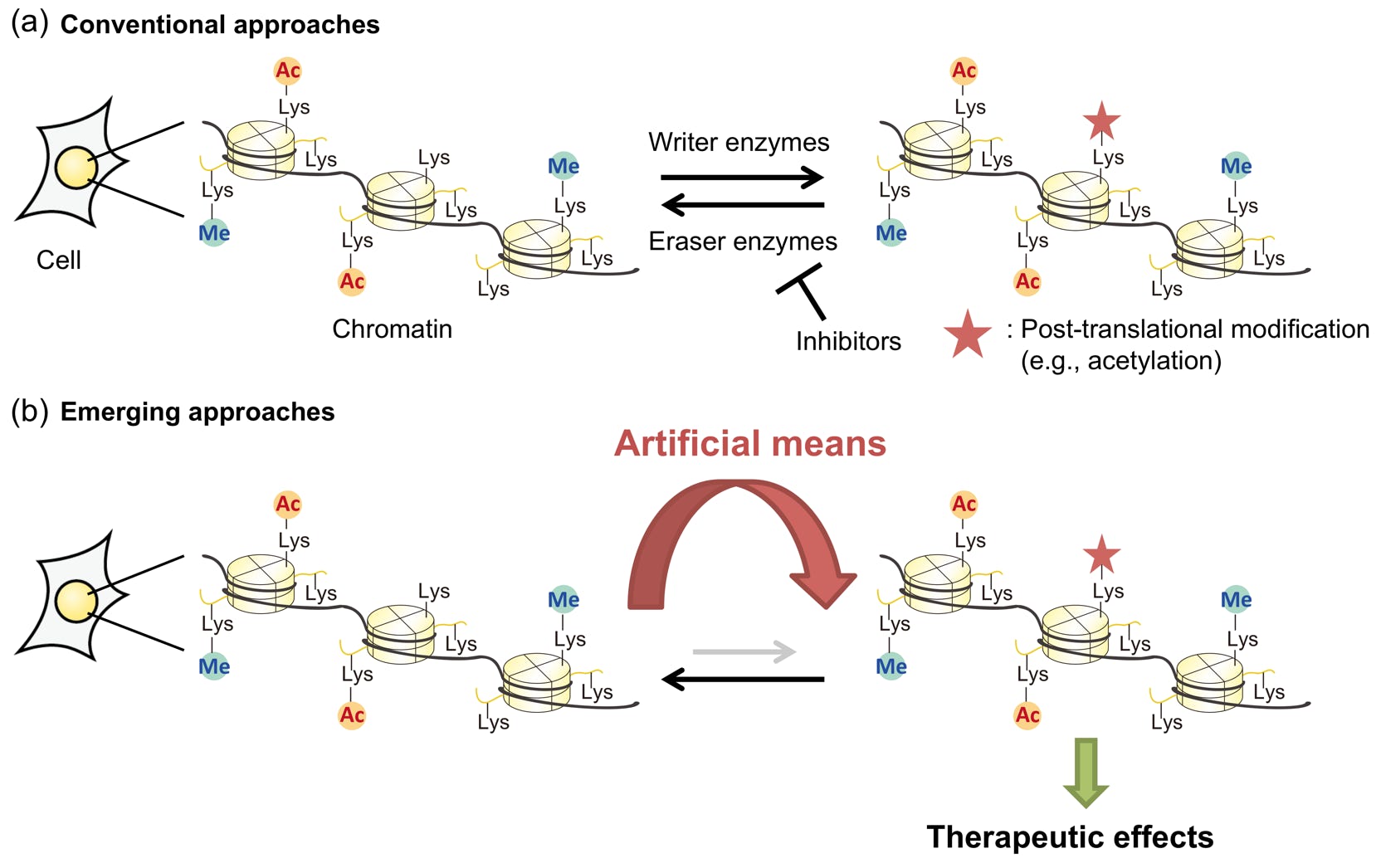
Yamatsugu, K.*; Kawashima, S. A.*; Kanai, M.*
“Leading approaches in synthetic epigenetics for novel therapeutic strategies”
Curr. Opin. Chem. Biol. 2018, 46, 10-17.
22.
Tanabe, K.; Liu, J.; Kato, D.; Kurumizaka, H.; Yamatsugu, K.; Kanai, M.*; Kawashima, S. A.*
“LC-MS/MS-based quantitative study of the acyl group- and site-selectivity of human sirtuins to acylated nucleosomes”
Sci. Rep. 2018, 8, 2656.
21.
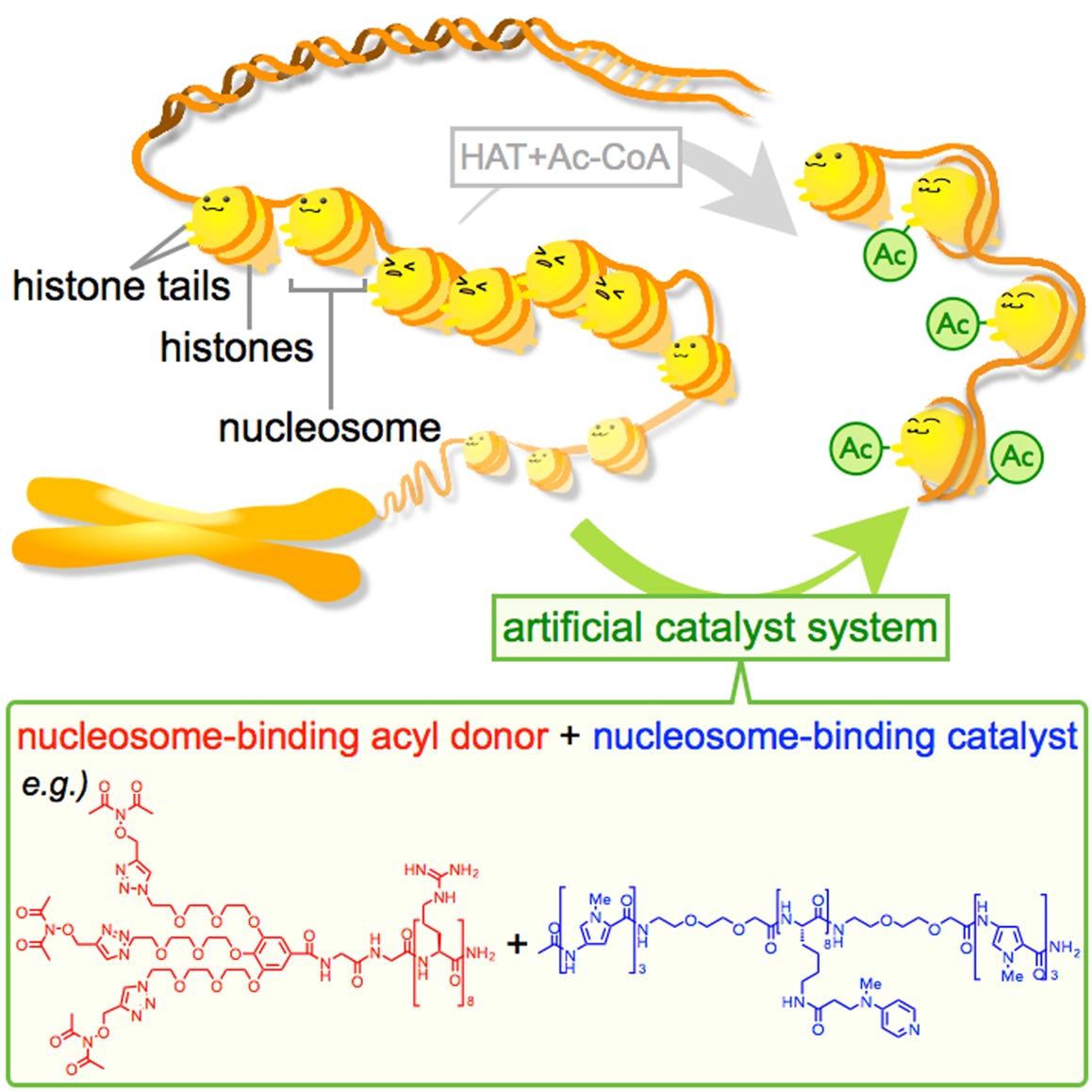
Ishiguro, T.; Amamoto, Y.; Tanabe, K.; Liu, J.; Kajino, H.; Fujimura, A.; Aoi, Y.; Osakabe, A.; Horikoshi, N.; Kurumizaka, H.; Yamatsugu, K.; Kawashima, S. A.*; Kanai, M.*
“Synthetic Chromatin Acylation by an Artificial Catalyst System”
Chem 2017, 2, 6, 840-859.
20.
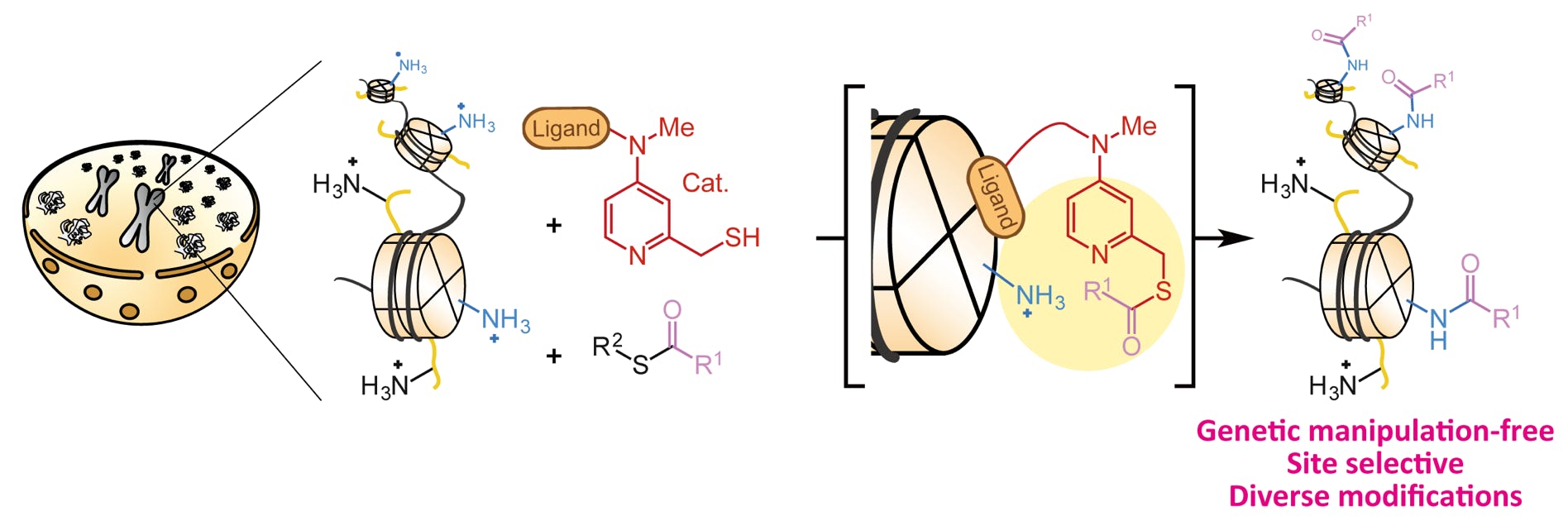
Amamoto, Y.; Aoi, Y.; Nagashima, N.; Suto, H.; Yoshidome, D.; Arimura, Y.; Osakabe, A.; Kato, D.; Kurumizaka, H.; Kawashima, S. A.; Yamatsugu, K.*; Kanai, M.*
“Synthetic Posttranslational Modifications: Chemical Catalyst-Driven Regioselective Histone Acylation of Native Chromatin”
J. Am. Chem. Soc. 2017, 139, 22, 7568-7576.
DOI: 10.1021/jacs.7b02138
19.
Yamatsugu, K.; Splain, R. A; Kiessling, L. L.*
“Fidelity and Promiscuity of a Mycobacterial Glycosyltransferase”
J. Am. Chem. Soc. 2016, 138, 29, 9205-9211.
DOI: 10.1021/jacs.6b04481

18.
Takemoto, A.*; Kawashima, S. A.; Li, J.-J.; Jeffery, L.; Yamatsugu, K.; Elemento, O.; Nurse, P.
“Nuclear envelope expansion is crucial for proper chromosomal segregation during a closed mitosis”
J. Cell Sci. 2016, 129, 1250-1259.
DOI: 10.1021/jacs.6b04481
17.
Kimura, Y.; Saito, N.; Hanada, K.; Liu, J.; Okabe, T.; Kawashima, S. A.*; Yamatsugu, K.*; Kanai, M.*
“Supramolecular Ligands for Histone Tails by Employing a Multivalent Display of Trisulfonated Calix[4]arenes”
ChemBioChem 2015, 16, 18, 2599-2604.

16.
Alagiri, K.; Furutachi, M.; Yamatsugu, K.; Kumagai, N.; Watanabe, T.*; Shibasaki, M.*
“Two Approaches toward the Formal Total Synthesis of Oseltamivir Phosphate (Tamiflu): Catalytic Enantioselective Three-Component Reaction Strategy and l-Glutamic Acid Strategy”
J. Org. Chem. 2013, 78, 8, 4019-4026.
DOI: 10.1021/jo400360j
15.
Komatsu, H.; Shindo, Y.; Kawashima, S. A.; Yamatsugu, K.; Oka K.; Kanai, M.*
“Intracellular activation of acetyl-CoA by an artificial reaction promoter and its fluorescent detection”
Chem. Commun. 2013, 49, 28, 2876-2878.
DOI: 10.1039/C3CC40616D
14.
Shibasaki, M.*; Kanai, M.; Yamatsugu, K.
“Recent Development in Synthetic Strategies for Oseltamivir Phosphate”
Israel J. Chem. 2011, 51, 3-4, 316-328.
13.
Kimura, Y.; Yamatsugu, K.; Kanai, M.*; Echigo, N.; Kuzuhara, T.; Shibasaki, M.*
“Design and Synthesis of Resin-Conjugated Tamiflu Analogs for Affinity Chromatography”
Bull. Korean. Chem. Soc. 2010, 31, 3, 588-594.
12.
Yamatsugu, K.; Kanai, M.*; Shibasaki, M.*
“An alternative synthesis of Tamiflu: a synthetic challenge and the identification of a ruthenium-catalyzed dihydroxylation route”
Tetrahedron 2009, 65, 31, 6017-6024.
11.
Tomita, D.; Yamatsugu, K.; Kanai, M.*; Shibasaki, M.*
“Enantioselective synthesis of SM-130686 based on the development of asymmetric Cu(I)F catalysis to access 2-oxindoles containing a tetrasubstituted carbon”
J. Am. Chem. Soc. 2009, 131, 20, 6946-6948.
DOI: 10.1021/ja901995a
10.
Kimura, Y.; Yamatsugu, K.; Kanai, M.*; Echigo, N.; Kuzuhara, T.; Shibasaki, M.*
“Design and synthesis of immobilized Tamiflu analog on resin for affinity chromatography”
Tetrahedron Lett. 2009, 50, 26, 3205-3208.
9.
Yamatsugu, K.; Yin, L.; Kamijo, S.; Kimura, Y.; Kanai, M.*; Shibasaki, M.*
“A synthesis of Tamiflu based on a barium-catalyzed Diels-Alder-type reaction”
Angew. Chem. Int. Ed. 2009, 48, 6, 1070-1076.
8.
Ose, A.; Ito, M.; Kusuhara, H.; Yamatsugu, K.; Kanai, M.; Shibasaki, M.; Hosokawa, M.; Schuetz, J. D.; Sugiyama, Y.*
“Limited brain distribution of Ro 64-0802, a pharmacologically active form of oseltamivir, by active efflux across the blood–brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4)”
Drug. Metab. Dispos. 2009, 37, 2, 315-321.
7.
Usami, A.; Sasaki, T.; Satoh, N.; Akiba, T.; Yokosima, S.; Fukuyama, T.; Yamatsugu, K., Kanai, M.; Shibasaki, M.; Matsuki, N.; Ikegaya, Y.*
“Oseltamivir enhances hippocampal network synchronization”
J. Pharmacol. Sci. 2008, 106, 4, 659-662.
6.
Ose, A.; Kusuhara, H.; Yamatsugu, K.; Kanai, M.; Shibasaki, M.; Fujita, T.; Yamamoto, A.; Sugiyama, Y.*
“P-glycoprotein restricts the penetration of oseltamivir across the blood-brain barrier”
Drug. Metab. Dispos. 2008, 36, 2, 427-434.
5.
Ishii, K.; Hamamoto, H.; Sasaki, T.; Ikegaya, Y.; Yamatsugu, K.; Kanai, M.; Shibasaki, M.; Sekimizu, K.*
“Pharmacologic action of oseltamivir on the nervous system”
Drug Discoveries & Therapeutics 2008, 2, 1, 24-34.
4.
Morita, M.; Sone, T.; Yamatsugu, K.; Sohtome, Y.; Matsunaga, S.; Kanai, M.*; Yasuyosi, W.; Shibasaki, M.*
“A method for the synthesis of an oseltamivir PET tracer”
Bioorg. Med. Chem. Lett. 2008, 18, 2, 600-602.
3.
Yamatsugu, K.; Kamijo, S.; Suto, Y.; Kanai, M.*; Shibasaki, M.*
“A concise synthesis of Tamiflu: third generation route via the Diels-Alder reaction and the Curtius rearrangement”
Tetrahedron Lett. 2007, 48, 8, 1403-1406.
2.
Yamatsugu, K.; Motoki, R.; Kanai, M.*; Shibasaki, M.*
“Identification of potent, selective protein kinase C inhibitors based on a phorbol skeleton”
Chem. Asian. J. 2006, 1, 3, 314-321.
1.
Kuramochi, A.; Usuda, H.; Yamatsugu, K.; Kanai, M.*; Shibasaki, M.*
“Total synthesis of (+/−)-garsubellin A”
J. Am. Chem. Soc. 2005, 127, 41, 14200-14201.
DOI: 10.1021/ja055301t