Themes related to intercellular communication
Back to research thme list
1. Intercellular communication in cancer
Cancer cells have a remarkable ability to survive by changing their properties in response to their environment. This is thought to be made possible due to the development of some sensors which catch the surrounding environment (development of cell surface molecules), adaptability to the microenvironment (interaction with surrounding cells), and specific mechanisms to reprogram themselves (modification of intercellular/intracellular metabolism). Furthermore, the surrounding cells that come into contact with the transforming cancer cells may also gradually change their appearance and contribute to cancer progression.
Regulation of the microenvironment by intercellular Gap Junction (GJ)
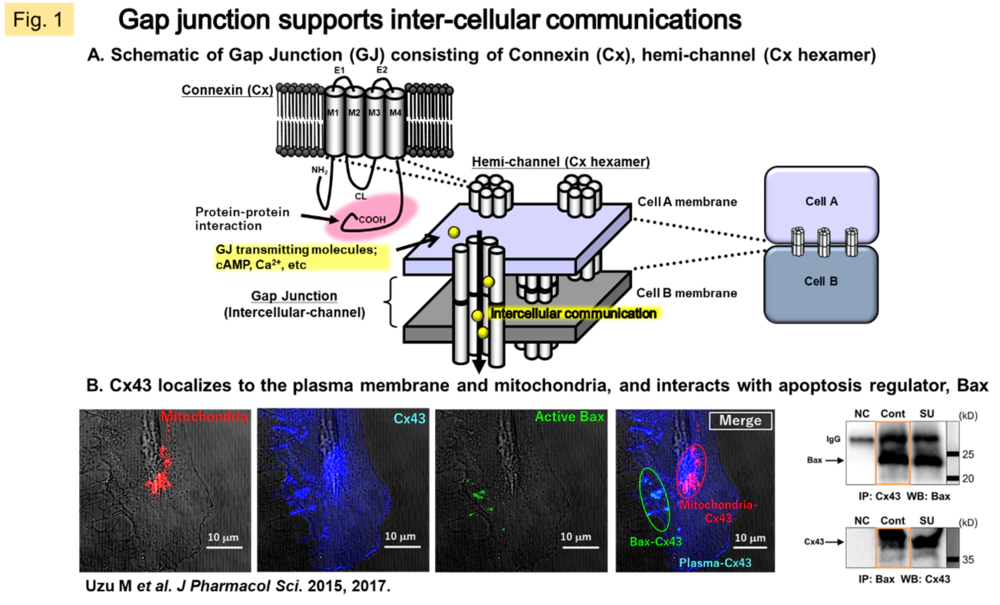
GJs, a form of intercellular junctions, also function as ion channels, allowing cytoplasmic components to pass directly between neighboring cells. The connexin (Cx) proteins make up hexamers that migrate to the plasma membrane to form unilateral channels (hemichannels), further, two hemichannels from neighboring cells build up gap junction (GJ). It has been demonstrated that GJs are permeable to nutrients such as glucose and glutamate as well as signaling molecules such as Ca2+, ATP, and cAMP (Fig. 1A). In humans, 21 molecular species of Cx have been identified, and while they are ubiquitous in cells throughout the body, it is considered that two or three molecular species are mainly expressed and function in each tissue, strictly regulating the spatiotemporal permeability of intercellular communication molecules.
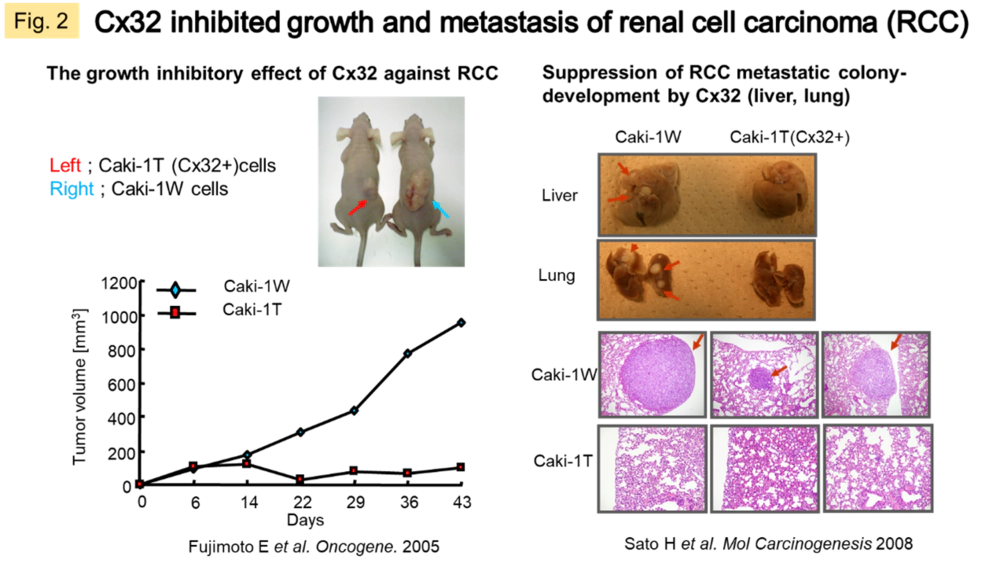
In general, Cx is an indicator of differentiated cells. There are also many reports that it fluctuates in certain pathological conditions, therefore taken as a response factor to stress, a disease susceptibility factor, etc. In cancer tissues, there is often a loss of Cx expression and function. We have confirmed that gene transfer of Cx32 or Cx43 into solid tumors such as lung cancer, renal cancer, and malignant mesothelioma causes inhibition of growth, reduction of metastatic and invasive potential, and improvement of drug sensitivity in human cancer cells and animal models (Fig. 2). This mechanism of action is suggested to be due to the restoration of GJ-mediated signal transduction in tissues or the interaction with other proteins in the carboxy-terminal region of Cx proteins (an intracytoplasmic region specific to Cx molecular species) (Fig. 1B). In a recent study, we searched for cytoplasmic molecules that directly interact with Cx (c-Src, Bax), and revealed a mechanism by which Cx43 activates the cytoplasmically localized apoptosis-inducing factor Bax, thereby promoting subsequent mitochondrial migration of Bax in response to sunitinib resistance in malignant mesothelioma.
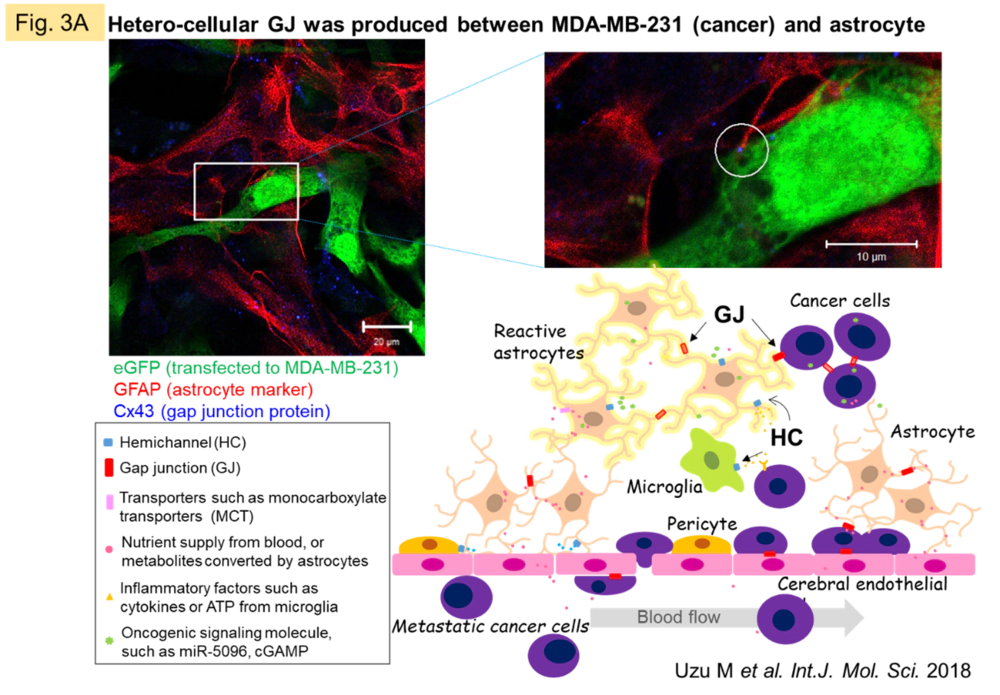
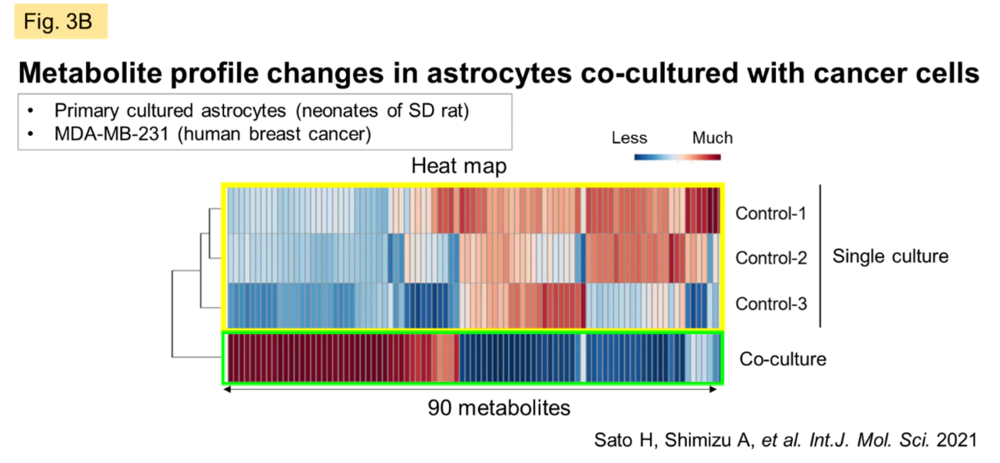
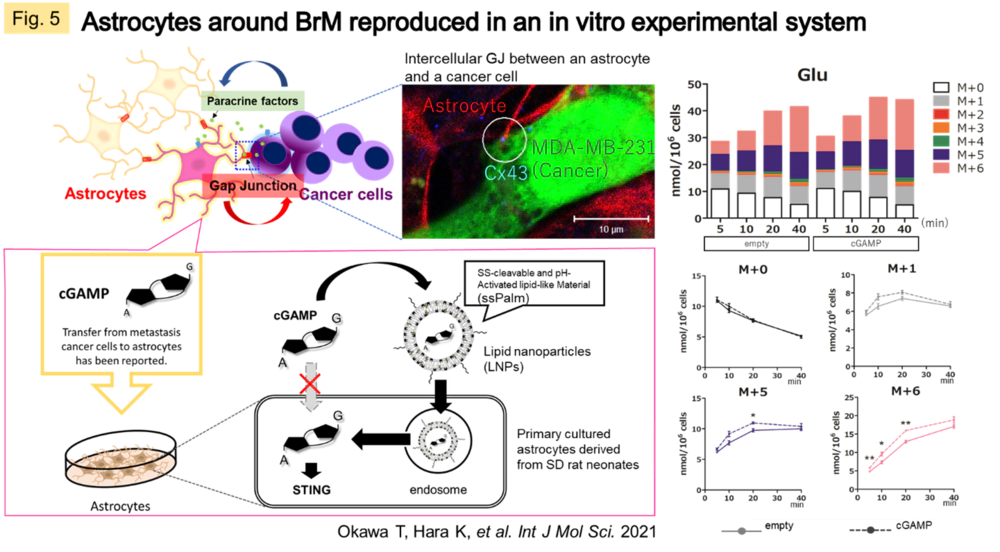
Despite the fact that the molecular mechanisms of Cx have been elucidated, the essential significance of GJ in vivo remains to be clarified. We believe that the answer to this question lies in understanding the dynamic network of GJs, patiently clarifying the molecules that permeate GJs, and the events/pathologies involved with fluctuations of GJs. Recently, we have been focusing on the central nervous system (CNS), where GJs are very active and their activity is easily maintained in vitro. In the CNS as a whole, GJ networks are observed in a variety of cell types, but are particularly prevalent in astrocytes, which are thought to occupy the majority of the brain parenchyma. In some types of gliomas (primary brain tumors with malignant neuronal and glial components), GJ function is reduced in proportion to the grade of the tumor. In metastatic brain tumors, however, GJs are formed between heterogeneous cells of metastatic carcinoma and astrocytes (Fig. 3A), and permeation of molecules from carcinoma cells may trigger communication between the two cells. We observed a significant change in the metabolic profile of astrocytes when cancer cells and astrocytes were mixed in culture compared to astrocytes alone or when cancer cell supernatants were added to astrocytes in culture (Fig. 3B). We are currently working on elucidating the nature of these interactions.
Elucidation of the mechanism of cancer drug resistance by multilayered analysis focusing on metabolism
In order to keep pace with their rapid growth rate, and sometimes to survive in a dormant state, cancer cells develop unique energy acquisition pathways. These changes can alter cellular traits in a relatively short period of time and are associated with events that can lead to cancer progression and failure of therapeutic agents. It is also reported that metabolic flexibility involves epigenetic regulation and that changes in the metabolome and epigenome are bidirectional. The following strategies will be the key to understanding the essential pathology and developing new therapeutic targets: where and how cancer trait changes are switched on and regulated, how they are related to proto-oncogenes, and how they propagate to surrounding cells. In order to connect the information in each layer, it is also essential to have a mechanism to realize relative quantification of data within each layer and between different phases. To this end, we are practicing quantitative systems pharmacology (QSP) by combining experimental data and accumulated existing information to elucidate the mechanisms of resistance across cancer types.
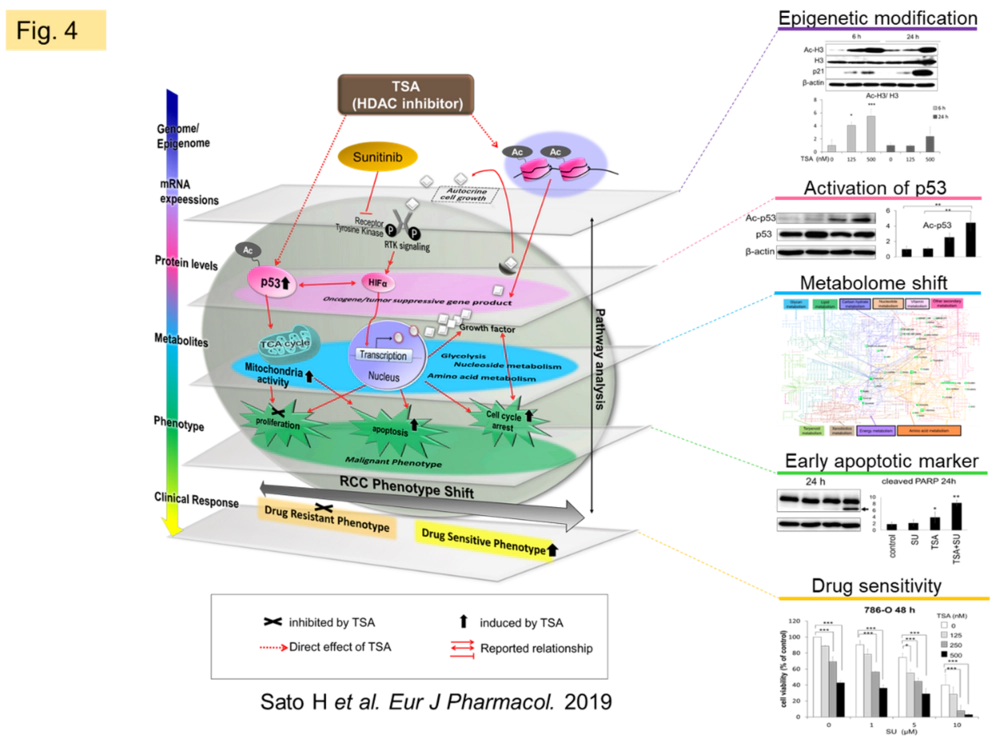
We confirmed that trichostatin A (TSA), a kind of epigenetic regulator, improves susceptibility of renal cell carcinoma to sunitinib, and showed that TSA has metabolic modulating effects in addition to its well-known gene modulating effects through histone deacetylase (HDAC) inhibitory activity (Fig. 4). We are currently investigating labeling studies to quantify the metabolite shift.
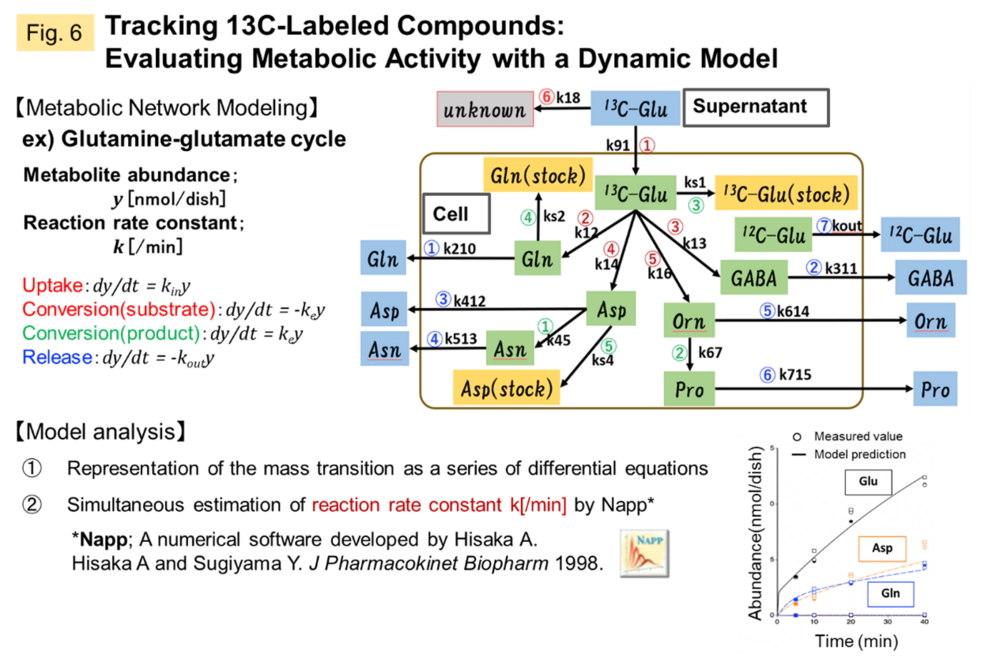
In order to understand the cancer microenvironment of metastatic brain tumors (BrM), we are currently working on quantifying the metabolomic shift of astrocytes, which are responsible for CNS homeostasis, upon stimulation from BrMs which come into contact with, and incorporating this information into QSP analysis. For this purpose, we have established an evaluation system that directly introduces 2'3'-cyclic GMP-AMP (cGAMP), a second messenger transmitted from cancer cells, into astrocytes, and are quantitatively evaluating metabolic activity using 13C-labeled compounds (Fig. 5, 6).
2. Intercellular communication in chronic diseases: diabetes and heart failure
Common mechanism of diabetes and ischemic heart disease; estimated from the action of SGLT2 inhibitors
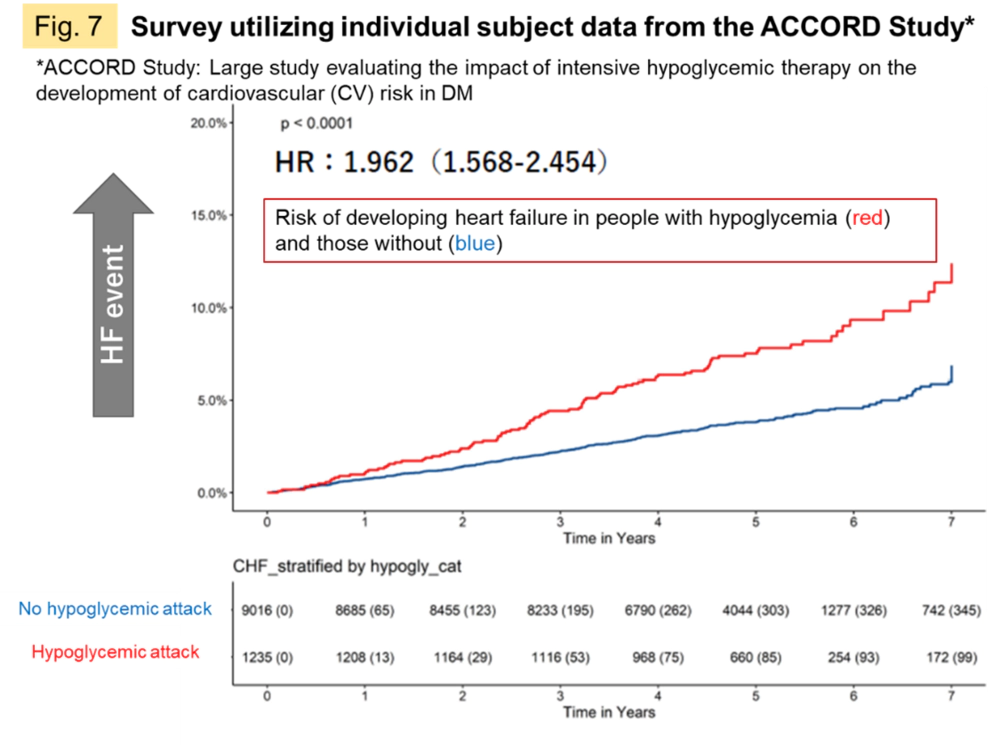
The progression of type 2 diabetes mellitus (DM) is associated with increased susceptibility to life-threatening cardiovascular (CV) events and chronic heart failure (HF). 60% of patients with HF are reported to have insulin resistance, a preliminary sign of DM. On the other hand, hypoglycemic agents and SGLT2 inhibitors, which were developed for the treatment of DM, have been shown to suppress CV events in DM and to prevent HF. It is hoped that the elucidation of pathomechanisms may lead to innovative and more comprehensive therapies to replace those that have been developed for DM as a separate disease. We examined individual subject information from clinical trials obtained from public repositories and confirmed that the risk of developing heart failure tends to increase after experiencing a hypoglycemic attack, a severe blood glucose fluctuation (Fig. 7), suggesting a common pathological mechanism.
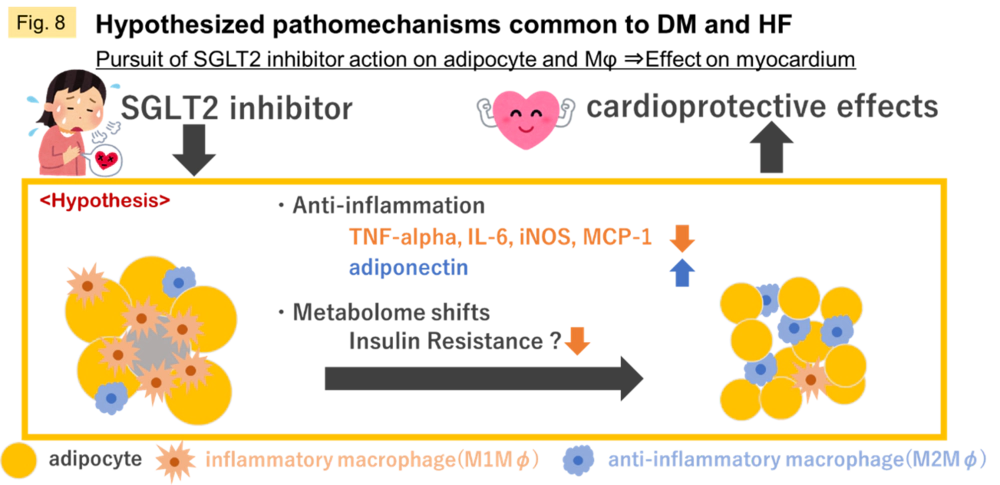
Currently, we are focusing on the inflammatory environment induced by adipocytes, macrophage mobilization, and metabolic shift in the myocardium as the main pathogenesis of ischemic heart disease progressing from DM (Fig. 8).
Research Approach
The interactions that occur between cells, their transmission into cells, and the physiological phenomena that occur (trait abnormalities) are supposed to be continuous, but only a snapshot of the whole process can be captured by technology. In recent years, significant advances in omics analysis have greatly lowered the threshold for comprehensive collection of information. Individual pieces of information may be fleeting, but in the aggregate, they are twice as powerful. On the other hand, data-driven analysis technology to connect information and logical thinking to pursue the real factors will be of great importance in the future, more and more. The process of carefully digesting raw data from all angles and diagonally, and developing interpretations through trial and error, is a bit painful but refreshing as well. I hope that researchers in this field will always be conscious of medical care, and at the same time be humble in your pursuit of natural science. I would like to work with everyone involved in this research in a friendly manner, believing in our own growth, while enjoying the dialogue with these chatty cells above all else.